
PhotoSound will be exhibiting at the upcoming BIOS Exhibition during the Photonics West conference. Meet Sam and Peter at Booth 8559 on January 17th and 18th at the Moscone Center in San Francisco

PhotoSound will be exhibiting at the upcoming BIOS Exhibition during the Photonics West conference. Meet Sam and Peter at Booth 8559 on January 17th and 18th at the Moscone Center in San Francisco
Author(s): Chuqin Huang a, Yanda Cheng a, Xiaoyu Zhang b, Ye Zhan c, Wenhan Zheng a, Isabel Komornicki d, Linda M. Harris d, Wenyao Xu b, Jun Xia a
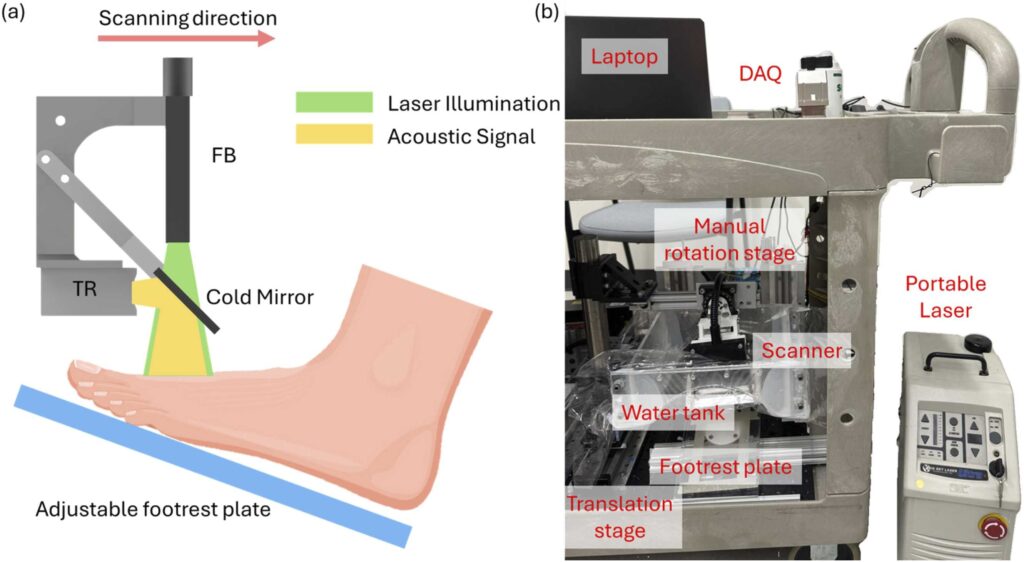
Accurate assessment of tissue perfusion is essential for managing chronic foot ulcers in patients with diabetes and peripheral arterial disease. While photoacoustic (PA) imaging enables high-resolution visualization of vascular structures, current perfusion evaluation methods are limited. We propose a fully automated radiomics-based framework for predicting perfusion conditions using single-wavelength clinical PA foot imaging. Radiomics features were extracted from both raw radiofrequency (RF) signals and reconstructed maximum amplitude projection (MAP) images. After reproducibility testing and statistical filtering, features were ranked using a combined minimum redundancy maximum relevance (mRMR) and ReliefF approach. A k-nearest neighbors ensemble model trained on eight selected features achieved an area under the curve (AUC) of 0.90 (training) and 0.94 (test). The selected features corresponded with physiological indicators such as vessel density, tissue structure, and vascular discontinuity. This study demonstrates a reliable and interpretable method for perfusion assessment in PA imaging with strong clinical potential.
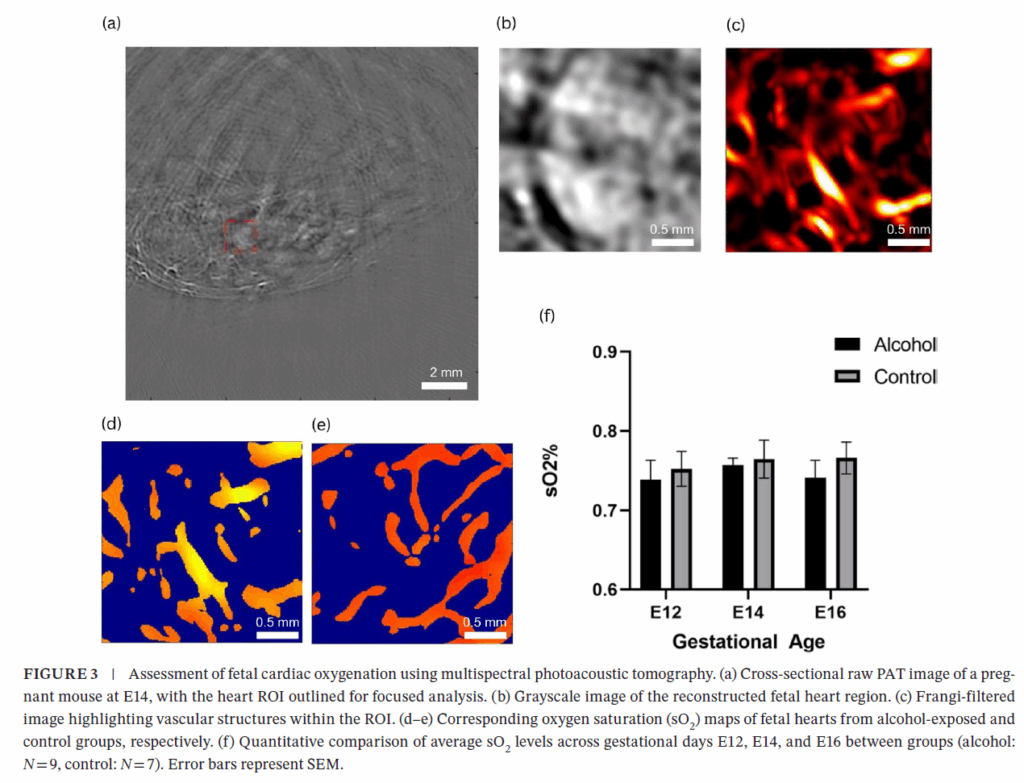
Prenatal alcohol exposure (PAE) is a leading cause of developmental abnormalities, yet its effects on fetal cardiac development remain understudied. We employed real-time, label-free
multispectral photoacoustic tomography (PAT) to noninvasively assess cardiac development in mouse fetuses exposed to chronic alcohol. Using a custom-built PAT system, fetal hearts were imaged from E12 to E16 in alcohol-exposed (3 g/kg ethanol via oral gavage, n = 9) and control (n = 7) CD-1 mice. PAT enabled quantitative measurements of cardiac morphology, oxygen saturation (sO2), and heart rate. Alcohol-exposed fetuses exhibited consistently lower sO2 and greater heart rate variability, particularly at later gestational stages. While structural growth progressed in both groups, functional impairments became more pronounced with alcohol exposure. These findings suggest PAE alters fetal cardiovascular regulation despite normal anatomical development. This study highlights the utility of PAT as a high-resolution, noninvasive tool for monitoring fetal cardiac health and supports its potential application in developmental biology and prenatal diagnostics.
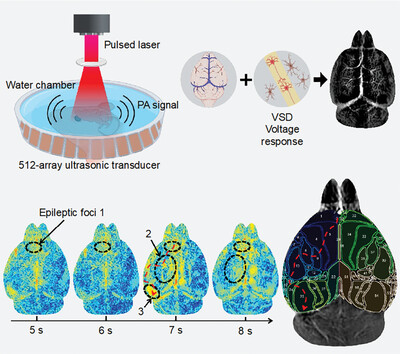
GRAPHICAL ABSTRACT
This work proposes a novel method for monitoring deep-brain electrodynamics with a voltage-sensitive dye (VSD)-based photoacoustic imaging platform, called PA-VSD. This platform is demonstrated to allow for effectively localizing epileptic foci and visualizing voltage conduction pathways in a high spatiotemporal resolution.
Brain voltage plays a crucial role in indicating internal functions or diseases, and optical voltage imaging has gained intensive attention in recent years. Despite encouraging progress, current implementations encounter limitations pertaining to penetration depth, field of view (FOV), and photostability of indicators. To mitigate these challenges, a robust voltage-sensitive dye (VSD)-based whole-field photoacoustic brain detection (WF-PABD) platform is proposed, enabling direct evaluation of voltage dynamics across the whole brain, forming as PA-VSD. WF-PABD is equipped with a 512-element ring-array ultrasound detector capable of 360-degree scanning, providing a large FOV (≈5 cm), high spatial resolution (≈110 µm), and rapid imaging acquisition. The proposed VSD remained ≈75% photostability after 30 min laser exposure, much greater than most calcium sensors. The optical voltage-response mechanisms are validated and the capability of PA-VSD to directly screen seizures is established. It is demonstrated that investigating connectivity among different brain regions allows to identify the precise location of active epileptic foci as well as the electrical conduction pathways and their directionality through fast temporal visualization. In summary, this study not only addresses the need for non-invasive, high-resolution, long-term, and direct monitoring of brain voltage but also empowers exciting venues for PA applications in neuroscience.
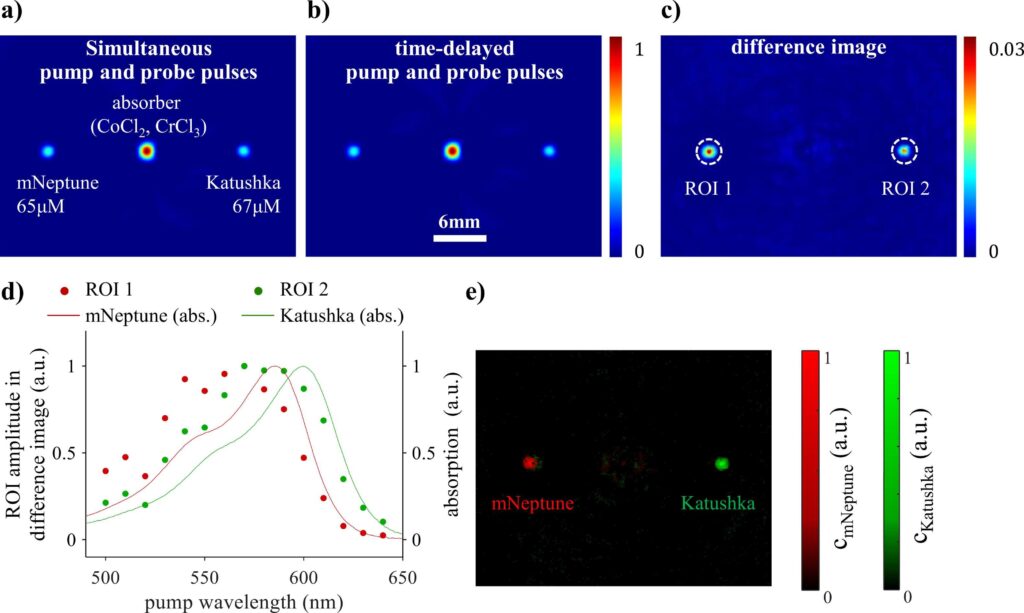
Pump-probe excitation of fluorophores has been shown to overcome the limitations of conventional multiwavelength imaging and linear unmixing approaches by providing fluorophore-specific contrast whilst eliminating the dominant background signal of endogenous chromophores. In this study, methods for generating pump-probe signals and images are investigated that rely on changing 1) the pump wavelength whilst keeping the probe wavelength fixed, 2) the probe wavelength whilst keeping the pump wavelength fixed, and 3) the time delay between the pump and probe pulse. Time-resolved PA signals were generated in purified solutions of genetically expressed red fluorescent proteins Katushka, mNeptune, and mCardinal in a cuvette. Spectra of the difference signal amplitude were found to correlate with the absorption and emission spectra. The difference signal plotted as a function of time delay also showed characteristic features for each protein. To demonstrate the capability of multiplexed imaging, the spatial distributions of Katushka and mNeptune were recovered from 2D difference images of a phantom. This study demonstrates that methods based on pump-probe excitation can be used to probe the photophysical properties of fluorophores. By detecting changes in these properties due to a stimulant, such as pH, the methods may find application in biosensing of the cellular microenvironment.
In this study, we utilized a home-made photoacoustic tomography system to recover optical absorption coefficient and elastic modulus under single wavelength. To reconstruct the optical absorption coefficient from photoacoustic measurements, we amalgamate the finite element solutions to the photoacoustic wave equation with MC simulation. Upon determining the absorption coefficient, the elastic modulus reconstruction relies on the analysis of the photoacoustic elastic tomography wave equation. In ex vivo trials, quantitative pencil lead and porcine liver tissue showed an absorption coefficient of 5015±5mm-1 and 0.236±0.01mm-1 at the wavelength of 800nm, respectively, while their elastic modulus parameters were 45.5±0.5GPa and 2.5±0.2GPa. Additionally, we implemented this methodology on in vivo three-dimensional quantitative finger imaging, obtaining the distribution of three-dimensional vascular absorption coefficients and elasticity modulus of the finger at the wavelength of 800nm. From these results, we confirm the method’s capability to discern differences in optical and acoustic properties between normal and abnormal tissues.
For ring-array ultrasound tomography, two-dimensional frequency-domain full waveform inversion is the clinical gold standard for high-resolution imaging of the breast. While yielding high-resolution images in the plane of the ring-array, the resulting slice-wise approach yields lower resolution out of plane when used to reconstruct the full volume. Instead, this work proposes a fully three-dimensional full-waveform inversion based on a multi-row ring-array transducer to improve out-of-plane resolution, while using cylindrical-wave transmissions to minimize acquisition and reconstruction time. For each numerical breast phantom tested, the root-mean-square error of three-dimensional full-waveform inversion is less than that of two-dimensional slice-wise full-waveform inversion by 6.3-13.7 m/s.
Author(s): Chuqin Huang, Emily Zheng, Wenhan Zheng, Huijuan Zhang, Yanda Cheng, Xiaoyu Zhang, Varun Shijo, Robert W. Bing, Isabel Komornicki, Linda M. Harris, Ermelinda Bonaccio, Kazuaki Takabe, Emma Zhang, Wenyao Xu, Jun Xia
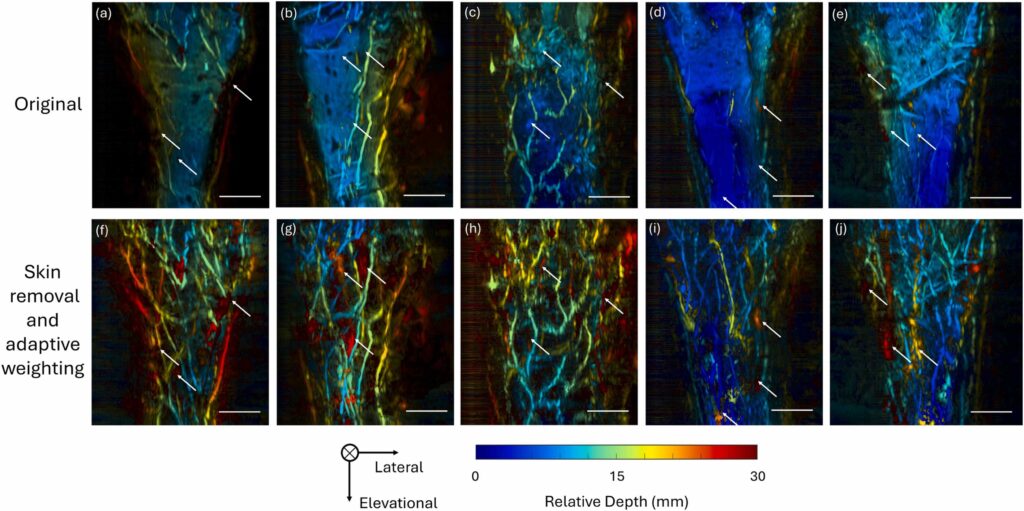
Photoacoustic tomography (PAT) is an emerging imaging modality with widespread applications in both preclinical and clinical studies. Despite its promising capabilities to provide high-resolution images, the visualization of vessels might be hampered by skin signals and attenuation in tissues. In this study, we have introduced a framework to retrieve deep vessels. It combines a deep learning network to segment skin layers and an adaptive weighting algorithm to compensate for attenuation. Evaluation of enhancement using vessel occupancy metrics and signal-to-noise ratio (SNR) demonstrates that the proposed method significantly recovers deep vessels across various body positions and skin tones. These findings indicate the method’s potential to enhance quantitative analysis in preclinical and clinical photoacoustic research.
Author(s): Zafar, Mohsin, Rayyan Manwar, Seyed Mohsen Ranjbaran, and Kamran Avanaki
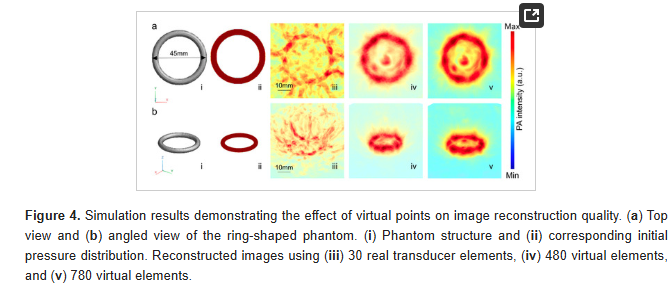
ABSTRACT
Typically, multi-single-element photoacoustic computed tomography (PACT) systems utilize numerous ultrasound transducers arranged in cylindrical or hemispherical configurations for detection, combined with a single diffuse light source or multiple sparse light sources to illuminate the imaging target. While these systems produce high-quality 3D PA images, they require complex, multi-channel data acquisition (DAQ) systems to acquire data from all transducers. These DAQ systems are often bulky and expensive, significantly limiting the clinical translation of PACT systems for patient care. In this study, we evaluated the feasibility of using a compact and cost-effective Texas Instruments analog front-end DAQ module for multi-single-element PACT systems. By imaging a simple 3D phantom, we demonstrated the capability of this affordable DAQ board, with reconstructed images showing promise for practical and economical solutions in PACT systems. This advancement paves the way for broader applications of PACT in both research and clinical settings.
Author(s): Daohuai Jiang, Hengrong Lan, Shangqing Tong, Xianzeng Zhang, Fei Gao
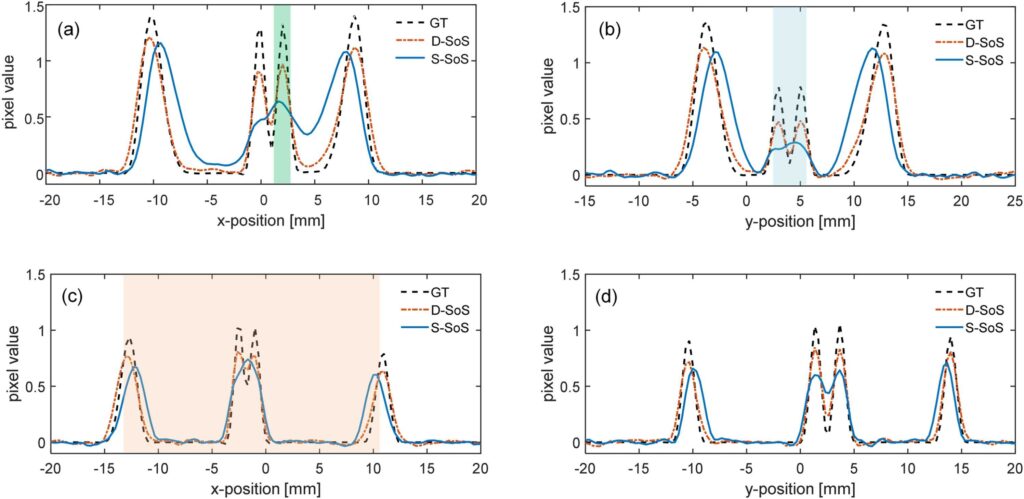
Photoacoustic imaging combines the advantages of optical and acoustic imaging, making it a promising tool in biomedical imaging. Photoacoustic tomography (PAT) reconstructs images by solving the inverse problem from detected photoacoustic waves to initial pressure map. The heterogeneous speed of sound (SoS) distribution in biological tissue significantly affects image quality, as uncertain SoS variations can cause image distortions. Previously reported dual-speed-of-sound (dual-SoS) imaging methods effectively address these distortions by accounting for the SoS differences between tissues and the coupling medium. However, these methods require recalculating the distribution parameters of the SoS for each frame during dynamic imaging, which is highly time-consuming and poses a significant challenge for achieving real-time dynamic dual-SoS PAT imaging. To address this issue, we propose a signal-domain dual-SoS correction method for PAT image reconstruction. In this method, two distinct SoS regions are differentiated by recognizing the photoacoustic signal features of the imaging target’s contours. The signals are then corrected based on the respective SoS values, enabling signal-domain-based dual-SoS dynamic real-time PAT imaging. The proposed method was validated through numerical simulations and in-vivo experiments of human finger. The results show that, compared to the single-SoS reconstruction method, the proposed approach produces higher-quality images, achieving the resolution error by near 12 times and a 30 % increase in contrast. Furthermore, the method enables dual-SoS dynamic real-time PAT reconstruction at 10 fps, which is 187.22 % faster than existing dual-SoS reconstruction methods, highlighting its feasibility for dynamic PAT imaging of heterogeneous tissues.