TriTomTM Contrast Agent Develpoment
The development of novel optical imaging contrast agents requires extensive testing in both phantoms and animal models to ensure the safety and utility of the agent for in vivo applications. However, evaluating the biodistribution and clearance of a contrast agent remains challenging in preclinical studies due to limited whole-body real-time imaging technologies. The TriTom is a multimodal imaging platform that provides high-resolution photoacoustic (PA) and fluorescence (FL) images of small animal models. Here, we demonstrate TriTom analysis of a novel photoacoustic agent and highlight the system’s unique advantages for advancing optical contrast agent development.

SYSTEM SPECIFICATIONS
Imaging System TriTomTM
Excitation Wavelengths 460 – 1300nm
Spatial Resolution Up to 160 µm(PA)
Up to 70 µm (FL)
Acquisition Time 36 s per scan
Contrast Agent Characterization
A critical step in developing novel photoacoustic contrast agents is determining the wavelength-dependent photoacoustic absorption spectrum. Indocyanine green (ICG) is a popular component of these agents, partly due to the ability to easily form J-aggregates (ICG-JA), which have increased stability and a stronger optical absorption in the NIR window. The TriTom provides high-resolution and high-sensitivity volumetric images of up to ten contrast agent samples in a single scan and requires minimal volumes (< 50 µL), which is beneficial for evaluating expensive or difficult-to-make contrast agents. Additionally, the ability to investigate multiple agents in a single scan allows for direct comparison to controls or other gold-standard references.
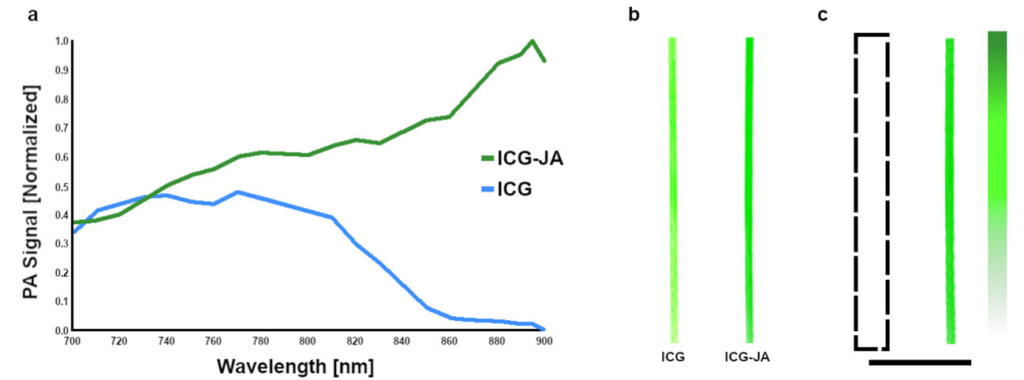
Data reproduced from [1].
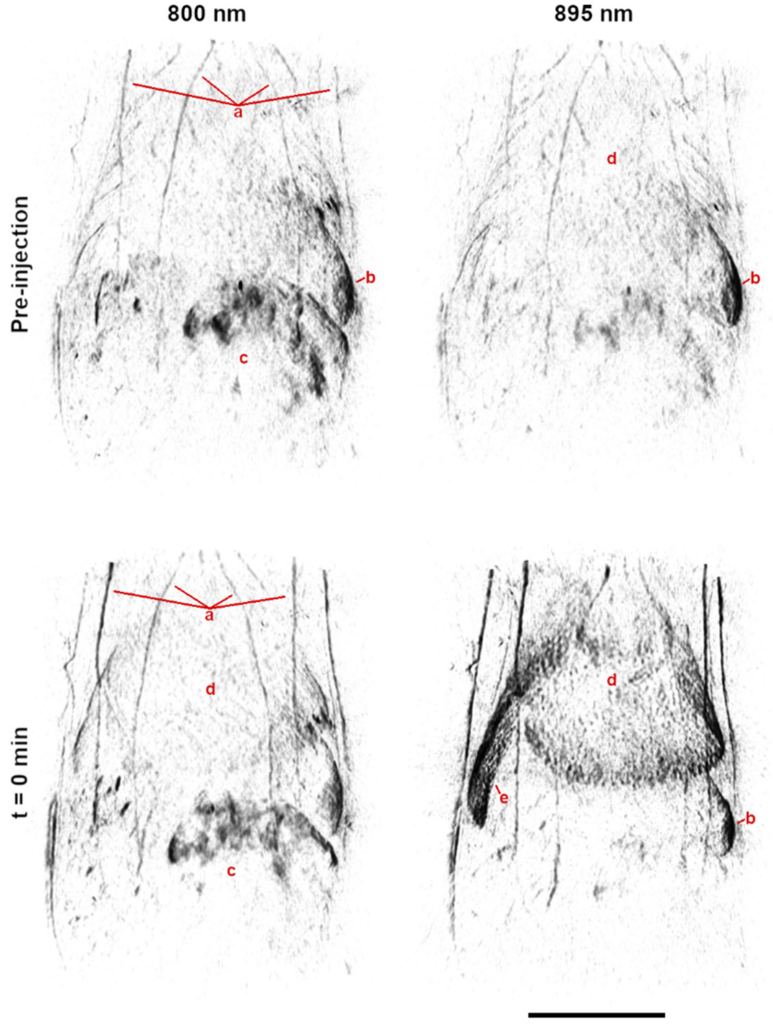
Contrast Biodistribution
Preclinical evaluation of the biodistribution and clearance of optical absorbers in healthy animal models are necessary to clinical translation of new contrast agents. However, these studies typically require large sample sizes or indirect measures due to inadequate tools for whole-body imaging. The TriTom provides high-resolution images of large volumes (> 30 cm3), enabling whole-body anatomical and molecular analysis of the contrast agent biodistribution. Further, the fast scan time (< 36 s) allows for functional imaging of the agent’s dynamics, physiologic interaction, and clearance mechanisms. These features allow for the direct, quantitative assessment of the agent in vivo, making the TriTom an ideal tool for developing novel optical contrast agents.

References
[1] S. Singh et al., Photoacoustics 29 (100437), doi: 10.1016/j.pacs.2022.100437 (2023).
